Introduction
Bioprocessing – the use of living cells or their components to produce valuable products such as biologic drugs, vaccines, enzymes and biofuels – has long been a cornerstone of biotechnology. Yet traditional bioprocess development and manufacturing often rely on time-consuming trial-and-error, expensive scale-up studies and manual monitoring. Enter artificial intelligence (AI). By bringing advanced data analytics, machine learning and real-time control together, AI-optimized bioprocessing is transforming how we design, monitor and scale these complex biological systems. This beginner’s guide will demystify the fundamentals, describe key methods, explore benefits and challenges, and highlight future directions in AI-driven biomanufacturing.
Definition
AI-Optimized Bioprocessing refers to the use of artificial intelligence technologies, such as machine learning and data analytics, to enhance and automate various stages of bioprocessing in biotechnology and pharmaceutical manufacturing. By analyzing large datasets from sensors and process parameters in real time, AI enables precise control, predictive maintenance, and process optimization, leading to improved efficiency, product quality, and reduced production costs.
Why Bioprocessing Matters
Bioprocessing underpins the manufacture of a huge range of products:
- Biopharmaceuticals. Monoclonal antibodies, vaccines and cell therapies now represent a multibillion-dollar industry.
- Industrial enzymes. Enzymes produced by microbes catalyze everything from laundry detergents to paper pulping.
- Biofuels and biochemicals. Engineered yeasts and bacteria convert biomass into ethanol, organic acids and specialty chemicals.
Despite its importance, bioprocessing remains more “art” than “science” in many organizations. Traditional process development typically involves:
- Design of Experiments (DoE) at small scale
- Manual sampling and off-line analytics
- Iterative optimization of culture conditions, media and feed rates
- Scale-up studies to pilots and manufacturing
These steps can take months or years, delaying product launches and driving up costs.
What Does “AI-Optimized” Mean?
AI-optimized bioprocessing leverages data science and machine learning to:
- Accelerate development. Build predictive models of cell growth and product formation to guide experiment design.
- Enhance monitoring. Use in-line sensors and computer vision to track critical quality attributes (CQAs) in real time.
- Enable advanced control. Deploy closed-loop systems that automatically adjust parameters—pH, dissolved oxygen, feed rates—based on AI predictions.
- Facilitate scale-up. Employ digital twins (virtual replicas of bioreactors) to predict large-scale performance from small-scale data.
In essence, AI turns bioprocess development into an iterative, data-driven cycle rather than a slow sequence of physical experiments.
Key AI Methods in Bioprocessing
Machine Learning Models:
Supervised learning algorithms (e.g., random forests, support vector machines, neural networks) can map relationships between input variables—temperature, nutrient feed, agitation speed—and outputs like cell density, metabolite concentrations or product titer. Once trained on historical DoE or run-data, these models predict optimal conditions, reducing the number of physical tests required.
Digital Twins:
A digital twin is a virtual representation of a physical bioprocess that integrates mechanistic (first-principles) models with AI-driven data. By running “what-if” simulations in silico, digital twins can forecast how changes in scale, reactor geometry or feed strategy will affect yields, thereby de-risking scale-up.
Process Analytical Technology (PAT) & Sensor Fusion:
PAT involves real-time measurement of CQAs using spectroscopic sensors (e.g., Raman, NIR), dielectric spectroscopy and other probes. AI algorithms fuse sensor streams to infer unmeasured variables—such as biomass concentration—and detect process drifts or contamination events faster than manual sampling.
Reinforcement Learning for Control:
Reinforcement learning (RL) agents learn optimal control policies by trial and error in simulation. In bioprocessing, an RL agent might adjust feed rates or pH setpoints continuously to maximize productivity, balancing trade-offs between cell health and product formation.
Benefits of AI-Optimized Bioprocessing
Faster Time to Market: Predictive modeling can cut DoE cycles by 30–50%, enabling quicker identification of optimal media and feeding strategies.
Improved Yields and Productivity: Closed-loop control maintains cells in their ideal physiological state, boosting titers and reducing batch variability.
Reduced Costs: Fewer physical experiments mean lower material and labor costs; digital twins minimize expensive scale-up failures.
Enhanced Quality Assurance: Continuous monitoring and anomaly detection improve compliance with regulatory expectations (e.g., FDA’s Quality by Design).
Data-Driven Knowledge Management: Centralized process data and AI models capture organizational learning, making it easier to transfer technology between facilities or products.
Challenges and Considerations
- Data Quality and Quantity. AI relies on robust datasets. Sparse, noisy or biased data can yield misleading models. Establishing standardized data pipelines and metadata annotation is crucial.
- Integration with Legacy Systems. Many biomanufacturing sites use stand-alone sensors and manual data capture. Upgrading to networked instrumentation and data historians requires capital investment.
- Regulatory Compliance. Agencies expect robust validation of AI algorithms. Demonstrating model interpretability, traceability and risk mitigation is essential for regulatory approval.
- Cross-Functional Expertise. Successful implementation demands collaboration between biologists, process engineers, data scientists and automation specialists.
Case Studies
Monoclonal Antibody Production:
A leading biopharma company implemented machine-learning models to predict viable cell densities and glycosylation profiles from Raman spectra. By integrating predictions into a model predictive control (MPC) framework, they increased antibody titer by 20% and reduced batch failure rates by 40%.
Industrial Enzyme Fermentation:
An enzyme manufacturer employed a digital twin combining a mechanistic metabolic model with neural-network corrections. Virtual scale-up allowed them to test nine scale scenarios in silico, selecting an optimal strategy that shortened pilot runs by six weeks, saving over $2 million in pilot plant costs.
Getting Started with AI-Driven Bioprocessing
Audit Your Data. Catalog existing historical process data, instrument logs and lab notebooks. Clean and standardize formats.
Define Objectives. Are you aiming to accelerate development, optimize an existing process or implement real-time control? Clear goals guide the choice of AI methods.
Pilot Small-Scale Implementations. Begin with a single unit operation (e.g., seed train) to validate AI predictions against observed results.
Invest in Infrastructure. Ensure you have reliable sensor networks, data storage and computing resources (on-premises or cloud).
Build Cross-Functional Teams. Bring together bioprocess experts, software engineers and data scientists. Foster a culture of experimentation and continuous learning.
Engage Early with Regulators. Share your AI-validation plans with regulatory bodies to align on expectations for model qualification and documentation.
The Future Outlook
AI-optimized bioprocessing is still in its early stages, but momentum is rapidly building:
- Autonomous Biomanufacturing. Advances in robotics + AI could one day enable fully autonomous “lights-out” facilities that develop, optimize and produce biologics with minimal human intervention.
- Generative Modeling. Just as AI can design small-molecule drugs, generative models may propose novel metabolic pathways or synthetic biology constructs to boost yields or create new bioproducts.
- Edge Computing & 5G. Real-time analytics at the edge will reduce latency in control loops, enabling ultra-fast responses to process disturbances.
- Digital Ecosystems. Shared digital twins and federated learning across organizations could accelerate innovation while preserving data privacy and intellectual property.
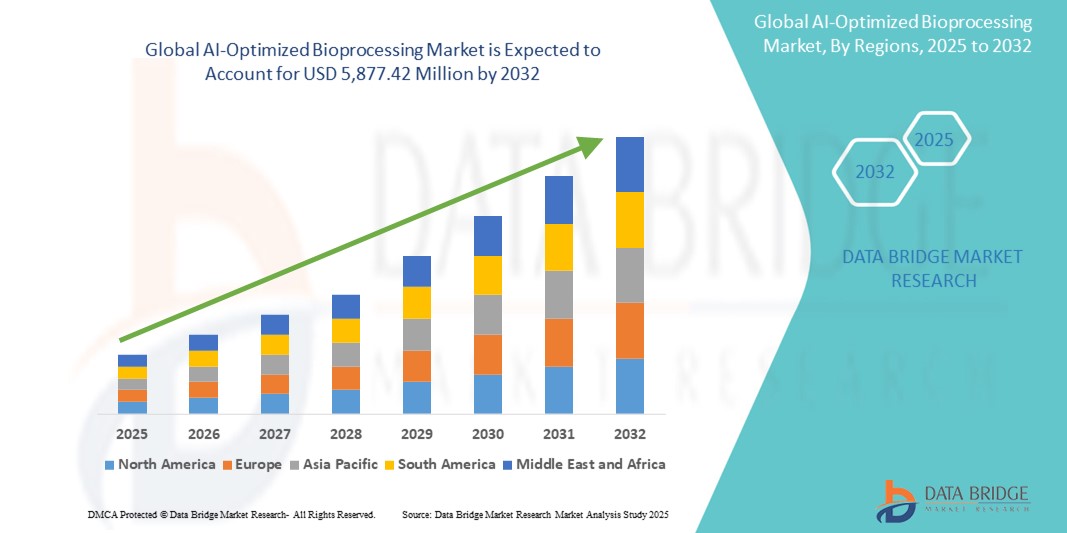
Growth Rate of AI-Optimized Bioprocessing Market
According to Data Bridge Market Research, the size of the global AI-optimized bioprocessing market was estimated at USD 841.60 million in 2024 and is projected to grow at a compound annual growth rate (CAGR) of 27.50% to reach USD 5,877.42 million by 2032.
Read More: https://www.databridgemarketresearch.com/reports/global-ai-optimized-bioprocessing-market
Conclusion
AI-optimized bioprocessing merges the best of biotechnology and data science to tackle longstanding challenges in process development, scale-up and manufacturing. By harnessing machine learning, digital twins, real-time analytics and advanced control strategies, organizations can bring products to market faster, at higher yields and with greater consistency. Although data quality, infrastructure and regulatory hurdles remain, a clear path exists for biomanufacturers to adopt AI systematically—starting small, validating rigorously, and scaling intelligently. For any biotech startup or established facility, now is the time to embrace AI-driven workflows and unlock the next frontier in bioprocess efficiency and innovation.